Key Points
-
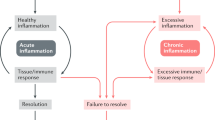
Inflammation is a beneficial process, designed to contain and eradicate threats to the host organism. Dysregulation of the magnitude or duration of inflammation contributes to multiple pathologies.
-
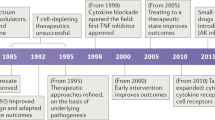
Traditionally, drugs have been designed to reduce inflammation. Some such approaches — for example, non-steroidal anti-inflammatory drugs or pro-inflammatory cytokine ablation — achieve this by targeting factors that drive inflammation; others — for example, glucocorticoids — are directly anti-inflammatory.
-
Active, specialized pathways bring about the resolution of inflammation. These involve discrete mediators and distinct cell phenotypes that act in a non-phlogistic manner to promote the clearance of inflammatory cells and a return to local tissue homeostasis.
-
Recent advances in our understanding of the central processes in the resolution of inflammation — including pro-inflammatory mediator catabolism, dampening of downstream signalling, apoptosis and efferocytosis of inflammatory cells and their regulation — permit targeted pharmacological interventions to promote inflammatory resolution.
-
The development of drugs that promote or mimic the mode of action of endogenous pro-resolution pathways may afford a novel complementary, or potentially superior, strategy to traditional options — thus regulating inflammation and restoring function, not merely suppressing inflammation.
-
Recent discoveries suggest that the innate immune response, and in particular its resolution, may modulate the subsequent development of adaptive immunity and so may afford further therapeutic targets. Multiple challenges remain in developing human models of inflammatory resolution and in translating murine discoveries to date into drugs for humans.
Abstract
Dysregulated inflammation is a central pathological process in diverse disease states. Traditionally, therapeutic approaches have sought to modulate the pro- or anti-inflammatory limbs of inflammation, with mixed success. However, insight into the pathways by which inflammation is resolved has highlighted novel opportunities to pharmacologically manipulate these processes — a strategy that might represent a complementary (and perhaps even superior) therapeutic approach. This Review discusses the state of the art in the biology of resolution of inflammation, highlighting the opportunities and challenges for translational research in this field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Buckley, C. D., Gilroy, D. W. & Serhan, C. N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 (2014).
Buckley, C. D., Gilroy, D. W., Serhan, C. N., Stockinger, B. & Tak, P. P. The resolution of inflammation. Nat. Rev. Immunol. 13, 59–66 (2013).
Gilroy, D. W. et al. Inducible cyclooxygenase-derived 15-deoxyΔ12–14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. Faseb J. 17, 2269–2271 (2003).
Newson, J. et al. Resolution of acute inflammation bridges the gap between innate and adaptive immunity. Blood 124, 1748–1764 (2014).
Nakano, H. et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat. Immunol. 10, 394–402 (2009).
Leon, B., Lopez-Bravo, M. & Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26, 519–531 (2007).
Wakim, L. M. & Bevan, M. J. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature 471, 629–632 (2011).
Ersland, K., Wuthrich, M. & Klein, B. S. Dynamic interplay among monocyte-derived, dermal, and resident lymph node dendritic cells during the generation of vaccine immunity to fungi. Cell Host Microbe 7, 474–487 (2010).
Uderhardt, S. et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity 36, 834–846 (2012). This paper very nicely differentiates between the role of tissue-resident macrophages versus monocyte-derived macrophages during the resolution of inflammation.
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Kool, M. et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 205, 869–882 (2008).
Wilson, E. B. et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207 (2013).
Teijaro, J. R. et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211 (2013). Papers 12 and 13 describe findings that inhibiting ongoing acute inflammation resulting from persistent lymphocytic choriomeningitis virus infection engages adaptive immunity and clears the infection, leading to resolution.
Gonzalez-Navajas, J. M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 (2012).
Boasso, A., Hardy, A. W., Anderson, S. A., Dolan, M. J. & Shearer, G. M. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS ONE 3, e2961 (2008).
Cope, A. P. et al. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J. Exp. Med. 185, 1573–1584 (1997).
Serhan, C. N. et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21, 325–332 (2007).
Wijbrandts, C. A. et al. Analysis of apoptosis in peripheral blood and synovial tissue very early after initiation of infliximab treatment in rheumatoid arthritis patients. Arthritis Rheum. 58, 3330–3339 (2008).
Thurlings, R. M. et al. Monocyte scintigraphy in rheumatoid arthritis: the dynamics of monocyte migration in immune-mediated inflammatory disease. PLoS ONE 4, e7865 (2009). References 18 and 19 illustrate the dynamics of monocyte trafficking in humans with rheumatoid arthritis and alludes to the possibility that dampening acute inflammation in this disease may also trigger resolution.
Mi, S. et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-β1-dependent and -independent mechanisms. J. Immunol. 187, 3003–3014 (2011).
Reddy, N. M., Potteti, H. R., Mariani, T. J., Biswal, S. & Reddy, S. P. Conditional deletion of Nrf2 in airway epithelium exacerbates acute lung injury and impairs the resolution of inflammation. Am. J. Respir. Cell. Mol. Biol. 45, 1161–1168 (2011).
Bynoe, M. S. et al. CD73 is critical for the resolution of murine colonic inflammation. J. Biomed. Biotechnol. 2012, 260983 (2012).
Mavers, M. et al. Cyclin-dependent kinase inhibitor p21, via its C-terminal domain, is essential for resolution of murine inflammatory arthritis. Arthritis Rheum. 64, 141–152 (2012).
Tomasini, R. et al. TAp73 is required for macrophage-mediated innate immunity and the resolution of inflammatory responses. Cell Death Differ. 20, 293–301 (2013).
Chiang, N. et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 (2012). This paper showed that specialized pro-resolving mediators greatly enhance antibiotic efficacy, and that the use of pro-resolution pharmacology to treat bacterial infection does not compromise host defence.
Favas, C. & Isenberg, D. A. B-cell-depletion therapy in SLE — what are the current prospects for its acceptance? Nat. Rev. Rheumatol. 5, 711–716 (2009).
Isenberg, D. A. Rituximab — it was the best of times, it was the worst of times. Autoimmun. Rev. 11, 790–791 (2012).
Segal, A. W., Geisow, M., Garcia, R., Harper, A. & Miller, R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature 290, 406–409 (1981).
Pollock, J. D. et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9, 202–209 (1995).
Stoecklin, G. & Anderson, P. Posttranscriptional mechanisms regulating the inflammatory response. Adv. Immunol. 89, 1–37 (2006).
Liu, J. et al. Identification and characterization of a unique leucine-rich repeat protein (LRRC33) that inhibits Toll-like receptor-mediated NF-κB activation. Biochem. Biophys. Res. Commun. 434, 28–34 (2013).
Divanovic, S. et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat. Immunol. 6, 571–578 (2005).
Kovalenko, A. et al. The tumour suppressor CYLD negatively regulates NF-κB signalling by deubiquitination. Nature 424, 801–805 (2003).
O'Brien, A. J. et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2 . Nat. Med. 20, 518–523 (2014).
Degraaf, A. J., Zaslona, Z., Bourdonnay, E. & Peters-Golden, M. Prostaglandin E2 reduces Toll-like receptor 4 expression in alveolar macrophages by inhibition of translation. Am. J. Respir. Cell. Mol. Biol. 51, 242–250 (2014).
Anderson, P. Post-transcriptional control of cytokine production. Nat. Immunol. 9, 353–359 (2008).
Carballo, E., Lai, W. S. & Blackshear, P. J. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood 95, 1891–1899 (2000).
Ogilvie, R. L. et al. Tristetraprolin down-regulates IL-2 gene expression through AU-rich element-mediated mRNA decay. J. Immunol. 174, 953–961 (2005).
Sauer, I. et al. Interferons limit inflammatory responses by induction of tristetraprolin. Blood 107, 4790–4797 (2006).
Linker, K. et al. Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic Acids Res. 33, 4813–4827 (2005).
Phillips, K., Kedersha, N., Shen, L., Blackshear, P. J. & Anderson, P. Arthritis suppressor genes TIA-1 and TTP dampen the expression of tumor necrosis factor α, cyclooxygenase 2, and inflammatory arthritis. Proc. Natl Acad. Sci. USA 101, 2011–2016 (2004).
Ogilvie, R. L. et al. Tristetraprolin mediates interferon-γ mRNA decay. J. Biol. Chem. 284, 11216–11223 (2009).
Carballo, E., Lai, W. S. & Blackshear, P. J. Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science 281, 1001–1005 (1998).
Chen, Y. L. et al. Differential regulation of ARE-mediated TNFα and IL-1β mRNA stability by lipopolysaccharide in RAW264.7 cells. Biochem. Biophys. Res. Commun. 346, 160–168 (2006).
von Roretz, C. & Gallouzi, I. E. Decoding ARE-mediated decay: is microRNA part of the equation? J. Cell Biol. 181, 189–194 (2008).
Shyu, A. B., Wilkinson, M. F. & van Hoof, A. Messenger RNA regulation: to translate or to degrade. EMBO J. 27, 471–481 (2008).
Piecyk, M. et al. TIA-1 is a translational silencer that selectively regulates the expression of TNF-α. EMBO J. 19, 4154–4163 (2000).
Muhl, H. & Pfeilschifter, J. Anti-inflammatory properties of pro-inflammatory interferon-γ. Int. Immunopharmacol. 3, 1247–1255 (2003).
Harvey, L. J. & McArdle, H. J. Biomarkers of copper status: a brief update. Br. J. Nutr. 99 (Suppl. 3), S10–S13 (2008).
Sampath, P., Mazumder, B., Seshadri, V. & Fox, P. L. Transcript-selective translational silencing by gamma interferon is directed by a novel structural element in the ceruloplasmin mRNA 3′ untranslated region. Mol. Cell. Biol. 23, 1509–1519 (2003).
Mukhopadhyay, R., Jia, J., Arif, A., Ray, P. S. & Fox, P. L. The GAIT system: a gatekeeper of inflammatory gene expression. Trends Biochem. Sci. 34, 324–331 (2009).
Vyas, K. et al. Genome-wide polysome profiling reveals an inflammation-responsive posttranscriptional operon in gamma interferon-activated monocytes. Mol. Cell. Biol. 29, 458–470 (2009).
Liang, J. et al. A novel CCCH-zinc finger protein family regulates proinflammatory activation of macrophages. J. Biol. Chem. 283, 6337–6346 (2008).
Matsushita, K. et al. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature 458, 1185–1190 (2009).
Taganov, K. D., Boldin, M. P., Chang, K. J. & Baltimore, D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl Acad. Sci. USA 103, 12481–12486 (2006).
Perry, M. M. et al. Rapid changes in microRNA-146a expression negatively regulate the IL-1β-induced inflammatory response in human lung alveolar epithelial cells. J. Immunol. 180, 5689–5698 (2008).
Bhaumik, D. et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY) 1, 402–411 (2009).
Jones, S. W. et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-α and MMP13. Osteoarthritis Cartilage 17, 464–472 (2009).
Nahid, M. A., Pauley, K. M., Satoh, M. & Chan, E. K. miR-146a is critical for endotoxin-induced tolerance: implication in innate immunity. J. Biol. Chem. 284, 34590–34599 (2009).
Sheedy, F. J. et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 11, 141–147 (2010).
Schmidt, M. F. Drug target miRNAs: chances and challenges. Trends Biotechnol. 32, 578–585 (2014).
Tili, E. et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 179, 5082–5089 (2007).
Bala, S. et al. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor α (TNFα) production via increased mRNA half-life in alcoholic liver disease. J. Biol. Chem. 286, 1436–1444 (2011).
Jones, M. R. et al. Zcchc11-dependent uridylation of microRNA directs cytokine expression. Nat. Cell Biol. 11, 1157–1163 (2009).
Hansen, W. R., Keelan, J. A., Skinner, S. J. & Mitchell, M. D. Key enzymes of prostaglandin biosynthesis and metabolism. Coordinate regulation of expression by cytokines in gestational tissues: a review. Prostaglandins Other Lipid Mediat. 57, 243–257 (1999).
Hahn, E. L. et al. Prostaglandin E2 alterations during sepsis are partially mediated by endotoxin-induced inhibition of prostaglandin 15-hydroxydehydrogenase. J. Trauma 44, 777–781; discussion 781–782 (1998).
Nibbs, R. J. & Graham, G. J. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 13, 815–829 (2013).
Ariel, A. et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat. Immunol. 7, 1209–1216 (2006).
Vanden Berghe, T., Linkermann, A., Jouan-Lanhouet, S., Walczak, H. & Vandenabeele, P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 15, 135–147 (2014).
Remijsen, Q. et al. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 18, 581–588 (2011).
Savill, J. Apoptosis in resolution of inflammation. J. Leukoc. Biol. 61, 375–380 (1997).
Cara, D. C., Negrao-Correa, D. & Teixeira, M. M. Mechanisms underlying eosinophil trafficking and their relevance in vivo. Histol. Histopathol. 15, 899–920 (2000).
Sousa, L. P. et al. Cyclic AMP enhances resolution of allergic pleurisy by promoting inflammatory cell apoptosis via inhibition of PI3K/Akt and NF-κB. Biochem. Pharmacol. 78, 396–405 (2009).
Lawrence, T. & Fong, C. The resolution of inflammation: anti-inflammatory roles for NF-κB. Int. J. Biochem. Cell Biol. 42, 519–523 (2010).
Song, G., Ouyang, G. & Bao, S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 9, 59–71 (2005).
Rodrigues, D. H. et al. Absence of PI3Kγ leads to increased leukocyte apoptosis and diminished severity of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 222, 90–94 (2010).
Insel, P. A., Zhang, L., Murray, F., Yokouchi, H. & Zambon, A. C. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol. (Oxf.) 204, 277–287 (2012).
Rossi, A. G. et al. Agents that elevate cAMP inhibit human neutrophil apoptosis. Biochem. Biophys. Res. Commun. 217, 892–899 (1995).
Rossi, A. G. et al. Regulation of macrophage phagocytosis of apoptotic cells by cAMP. J. Immunol. 160, 3562–3568 (1998).
Sousa, L. P. et al. PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in a PKA–PI3K/Akt-dependent and NF-κB-independent manner. J. Leukoc. Biol. 87, 895–904 (2010).
Bystrom, J. et al. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood 112, 4117–4127 (2008).
Junttila, M. R., Li, S. P. & Westermarck, J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 22, 954–965 (2008).
Chapman, M. S. & Miner, J. N. Novel mitogen-activated protein kinase kinase inhibitors. Expert Opin. Investig. Drugs 20, 209–220 (2011).
Sawatzky, D. A., Willoughby, D. A., Colville-Nash, P. R. & Rossi, A. G. The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-xL and Bax in the resolution of acute inflammation in vivo. Am. J. Pathol. 168, 33–41 (2006).
Langereis, J. D., Raaijmakers, H. A., Ulfman, L. H. & Koenderman, L. Abrogation of NF-κB signaling in human neutrophils induces neutrophil survival through sustained p38-MAPK activation. J. Leukoc. Biol. 88, 655–664 (2010).
Allaeys, I., Gymninova, I., Canet-Jourdan, C. & Poubelle, P. E. IL-32γ delays spontaneous apoptosis of human neutrophils through MCL-1, regulated primarily by the p38 MAPK pathway. PLoS ONE 9, e109256 (2014).
Perdiguero, E., Kharraz, Y., Serrano, A. L. & Munoz-Canoves, P. MKP-1 coordinates ordered macrophage-phenotype transitions essential for stem cell-dependent tissue repair. Cell Cycle 11, 877–886 (2012).
Chung, E. Y. et al. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity 27, 952–964 (2007).
Leitch, A. E. et al. Cyclin-dependent kinases 7 and 9 specifically regulate neutrophil transcription and their inhibition drives apoptosis to promote resolution of inflammation. Cell Death Differ. 19, 1950–1961 (2012).
Rossi, A. G. et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 12, 1056–1064 (2006). One of the first experimental papers showing that pharmacologically triggering PMN apoptosis leads to resolution.
Leitch, A. E. et al. The cyclin-dependent kinase inhibitor R-roscovitine down-regulates Mcl-1 to override pro-inflammatory signalling and drive neutrophil apoptosis. Eur. J. Immunol. 40, 1127–1138 (2010).
Alessandri, A. L. et al. Induction of eosinophil apoptosis by the cyclin-dependent kinase inhibitor AT7519 promotes the resolution of eosinophil-dominant allergic inflammation. PLoS ONE 6, e25683 (2011).
Shao, W. H. & Cohen, P. L. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res. Ther. 13, 202 (2011).
Brown, J. R., Goldblatt, D., Buddle, J., Morton, L. & Thrasher, A. J. Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD). J. Leukoc. Biol. 73, 591–599 (2003).
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009).
Lauber, K. et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113, 717–730 (2003).
Gude, D. R. et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 22, 2629–2638 (2008).
Knies, U. E. et al. Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proc. Natl Acad. Sci. USA 95, 12322–12327 (1998).
Horino, K. et al. A monocyte chemotactic factor, S19 ribosomal protein dimer, in phagocytic clearance of apoptotic cells. Lab. Invest. J. Techn. Methods Pathol. 78, 603–617 (1998).
Savill, J., Hogg, N., Ren, Y. & Haslett, C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 90, 1513–1522 (1992).
Schwab, J. M., Chiang, N., Arita, M. & Serhan, C. N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874 (2007).
Vago, J. P. et al. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J. Leukoc. Biol. 92, 249–258 (2012).
Brown, S. et al. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418, 200–203 (2002).
Gardai, S. J. et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123, 321–334 (2005).
Fadok, V. A., Bratton, D. L. & Henson, P. M. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J. Clin. Invest. 108, 957–962 (2001).
Gregory, C. D. & Pound, J. D. Microenvironmental influences of apoptosis in vivo and in vitro. Apoptosis 15, 1029–1049 (2010).
Park, D. et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434 (2007).
Devitt, A. et al. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392, 505–509 (1998).
Fadok, V. A. & Henson, P. M. Apoptosis: giving phosphatidylserine recognition an assist — with a twist. Curr. Biol. 13, R655–R657 (2003).
Bystrom, J., Wynn, T. A., Domachowske, J. B. & Rosenberg, H. F. Gene microarray analysis reveals interleukin-5-dependent transcriptional targets in mouse bone marrow. Blood 103, 868–877 (2004).
Bellingan, G. J. et al. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J. Exp. Med. 196, 1515–1521 (2002).
Ariel, A. & Serhan, C. N. New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front. Immunol. 3, 4 (2012).
Schif-Zuck, S. et al. Saturated-efferocytosis generates pro-resolving CD11blow macrophages: modulation by resolvins and glucocorticoids. Eur. J. Immunol. 41, 366–379 (2011).
Fadok, V. A., Warner, M. L., Bratton, D. L. & Henson, P. M. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (αvβ3). J. Immunol. 161, 6250–6257 (1998).
Fadok, V. A. & Henson, P. M. Apoptosis: getting rid of the bodies. Curr. Biol. 8, R693–R695 (1998).
Fadok, V. A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101, 890–898 (1998).
Serhan, C. N. et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 26, 1755–1765 (2012).
Medeiros, A. I., Serezani, C. H., Lee, S. P. & Peters-Golden, M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J. Exp. Med. 206, 61–68 (2009).
van Rijt, L. S. et al. Persistent activation of dendritic cells after resolution of allergic airway inflammation breaks tolerance to inhaled allergens in mice. Am. J. Respir. Crit. Care Med. 184, 303–311 (2011).
Rosas, M. et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science 344, 645–648 (2014).
Okabe, Y. & Medzhitov, R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157, 832–844 (2014).
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl Acad. Sci. USA 109, E3186–E3195 (2012). This paper emphasizes that monocyte or macrophage populations may possess diverse phenotypes that are neither M1 (pro-inflammatory) nor M2 (anti-inflammatory), but are commensurate with the phase of inflammation.
Anders, H. J. & Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 80, 915–925 (2011).
Arnold, L. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007). This paper demonstrates the effect of macrophage phenotype switching on tissue resolution.
Mounier, R. et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell. Metab. 18, 251–264 (2013).
Bannenberg, G. L. et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 (2005).
Zmijewski, J. W. et al. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am. J. Respir. Crit. Care Med. 178, 168–179 (2008).
Serhan, C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 (2014).
Alessandri, A. L. et al. Resolution of inflammation: mechanisms and opportunity for drug development. Pharmacol. Ther. 139, 189–212 (2013).
Poon, I. K., Lucas, C. D., Rossi, A. G. & Ravichandran, K. S. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 14, 166–180 (2014).
Koedel, U. et al. Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog. 5, e1000461 (2009).
Cash, J. L., Norling, L. V. & Perretti, M. Resolution of inflammation: targeting GPCRs that interact with lipids and peptides. Drug Discov. Today 19, 1186–1192 (2014).
Gilroy, D. W. et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 5, 698–701 (1999).
Rajakariar, R. et al. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyΔ12–14 PGJ2 . Proc. Natl Acad. Sci. USA 104, 20979–20984 (2007).
Trivedi, S. G. et al. Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proc. Natl Acad. Sci. USA 103, 5179–5184 (2006).
Chan, M. M. & Moore, A. R. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J. Immunol. 184, 6418–6426 (2010).
Straus, D. S. & Glass, C. K. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28, 551–558 (2007).
Rossi, A. et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 403, 103–108 (2000).
Kim, W. J., Kim, J. H. & Jang, S. K. Anti-inflammatory lipid mediator 15d-PGJ2 inhibits translation through inactivation of eIF4A. EMBO J. 26, 5020–5032 (2007).
Anderson, P. & Kedersha, N. Stress granules: the Tao of RNA triage. Trends Biochem. Sci. 33, 141–150 (2008).
Recchiuti, A., Krishnamoorthy, S., Fredman, G., Chiang, N. & Serhan, C. N. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 25, 544–560 (2011).
Krishnamoorthy, S., Recchiuti, A., Chiang, N., Fredman, G. & Serhan, C. N. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol. 180, 2018–2027 (2012).
Braley-Mullen, H. & Sharp, G. C. Adoptive transfer murine model of granulomatous experimental autoimmune thyroiditis. Int. Rev. Immunol. 19, 535–555 (2000).
Fang, Y., Sharp, G. C., Yagita, H. & Braley-Mullen, H. A critical role for TRAIL in resolution of granulomatous experimental autoimmune thyroiditis. J. Pathol. 216, 505–513 (2008).
Fang, Y., Sharp, G. C. & Braley-Mullen, H. Interleukin-10 promotes resolution of granulomatous experimental autoimmune thyroiditis. Am. J. Pathol. 172, 1591–1602 (2008).
McGrath, E. E. et al. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J. Leukoc. Biol. 90, 855–865 (2011).
Gemici, B. et al. H2S-releasing drugs: anti-inflammatory, cytoprotective and chemopreventative potential. Nitric Oxide 46, 25–31 (2014).
Dufton, N., Natividad, J., Verdu, E. F. & Wallace, J. L. Hydrogen sulfide and resolution of acute inflammation: a comparative study utilizing a novel fluorescent probe. Sci. Rep. 2, 499 (2012).
Wallace, J. L. & Wang, R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 14, 329–345 (2015).
Montero-Melendez, T. ACTH: The forgotten therapy. Semin. Immunol. 27, 216–226 (2015).
Matzelle, M. M. et al. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 64, 1540–1550 (2012).
Chiang, N., Dalli, J., Colas, R. A. & Serhan, C. N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 212, 1203–1217 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00799552 (2010).
Cahill, R. N., Frost, H. & Trnka, Z. The effects of antigen on the migration of recirculating lymphocytes through single lymph nodes. J. Exp. Med. 143, 870–888 (1976).
Hopkins, J., McConnell, I. & Pearson, J. D. Lymphocyte traffic through antigen-stimulated lymph nodes. II. Role of prostaglandin E2 as a mediator of cell shutdown. Immunology 42, 225–231 (1981).
McConnell, I. & Hopkins, J. Lymphocyte traffic through antigen-stimulated lymph nodes. I. Complement activation within lymph nodes initiates cell shutdown. Immunology 42, 217–223 (1981).
Johnston, M. G., Hay, J. B. & Movat, H. Z. Kinetics of prostaglandin production in various inflammatory lesions, measured in draining lymph. Am. J. Pathol. 95, 225–238 (1979).
Yang, C. W. & Unanue, E. R. Neutrophils control the magnitude and spread of the immune response in a thromboxane A2-mediated process. J. Exp. Med. 210, 375–387 (2013).
Yao, C. et al. Prostaglandin E2 promotes Th1 differentiation via synergistic amplification of IL-12 signalling by cAMP and PI3-kinase. Nat. Commun. 4, 1685 (2013).
Mitroulis, I., Kourtzelis, I., Kambas, K., Chrysanthopoulou, A. & Ritis, K. Evidence for the involvement of mTOR inhibition and basal autophagy in familial Mediterranean fever phenotype. Hum. Immunol. 72, 135–138 (2011).
Deretic, V., Saitoh, T. & Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13, 722–737 (2013).
Amulic, B. & Hayes, G. Neutrophil extracellular traps. Curr. Biol. 21, R297–R298 (2011).
Mitroulis, I. et al. Neutrophil extracellular trap formation is associated with IL-1β and autophagy-related signaling in gout. PLoS ONE 6, e29318 (2011).
Mocarski, E. S., Kaiser, W. J., Livingston-Rosanoff, D., Upton, J. W. & Daley-Bauer, L. P. True grit: programmed necrosis in antiviral host defense, inflammation, and immunogenicity. J. Immunol. 192, 2019–2026 (2014).
Berger, S. B. et al. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 192, 5476–5480 (2014).
Prince, L. R. et al. Staphylococcus aureus induces eosinophil cell death mediated by α-hemolysin. PLoS ONE 7, e31506 (2012).
Feoktistova, M. et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 (2011).
Chan, F. K. Fueling the flames: mammalian programmed necrosis in inflammatory diseases. Cold Spring Harb. Perspect. Biol. 4, a008805 (2012).
Miles, K. et al. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of α-defensins. J. Immunol. 183, 2122–2132 (2009).
Blume, K. E. et al. Cell surface externalization of annexin A1 as a failsafe mechanism preventing inflammatory responses during secondary necrosis. J. Immunol. 183, 8138–8147 (2009).
Menzies, F. M., Moreau, K. & Rubinsztein, D. C. Protein misfolding disorders and macroautophagy. Curr. Opin. Cell Biol. 23, 190–197 (2011).
Mihalache, C. C. et al. Inflammation-associated autophagy-related programmed necrotic death of human neutrophils characterized by organelle fusion events. J. Immunol. 186, 6532–6542 (2011).
Schauer, C. et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 20, 511–517 (2014).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 110, 3507–3512 (2013).
Takao, K. & Miyakawa, T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 112, 1167–1172 (2015).
Shay, T., Lederer, J. A. & Benoist, C. Genomic responses to inflammation in mouse models mimic humans: we concur, apples to oranges comparisons won't do. Proc. Natl Acad. Sci. USA 112, E346 (2015).
Libby, P., Tabas, I., Fredman, G. & Fisher, E. A. Inflammation and its resolution as determinants of acute coronary syndromes. Circ. Res. 114, 1867–1879 (2014).
Nicholls, S. J. et al. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 365, 2078–2087 (2011).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Drechsler, M., Megens, R. T., van Zandvoort, M., Weber, C. & Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122, 1837–1845 (2010).
Parathath, S. et al. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes 60, 1759–1769 (2011).
Day, R. M., Harbord, M., Forbes, A. & Segal, A. W. Cantharidin blisters: a technique for investigating leukocyte trafficking and cytokine production at sites of inflammation in humans. J. Immunol. Methods 257, 213–220 (2001).
Evans, B. J., Haskard, D. O., Sempowksi, G. & Landis, R. C. Evolution of the macrophage CD163 phenotype and cytokine profiles in a human model of resolving inflammation. Int. J. Inflam 2013, 780502 (2013).
Jenner, W. et al. Characterisation of leukocytes in a human skin blister model of acute inflammation and resolution. PLoS ONE 9, e89375 (2014).
Jenner, W. J. & Gilroy, D. W. Assessment of leukocyte trafficking in humans using the cantharidin blister model. JRSM Cardiovasc. Dis. http://dx.doi.org/10.1258/cvd.2012.012009 (2012).
Basran, A. et al. Roles of neutrophils in the regulation of the extent of human inflammation through delivery of IL-1 and clearance of chemokines. J. Leukoc. Biol. 93, 7–19 (2013).
Brittan, M. et al. A novel subpopulation of monocyte-like cells in the human lung after lipopolysaccharide inhalation. Eur. Respir. J. 40, 206–214 (2012).
Vukmanovic-Stejic, M., Rustin, M. H., Nikolich-Zugich, J. & Akbar, A. N. Immune responses in the skin in old age. Curr. Opin. Immunol. 23, 525–531 (2011).
High, K. P., Akbar, A. N. & Nikolich-Zugich, J. Translational research in immune senescence: assessing the relevance of current models. Semin. Immunol. 24, 373–382 (2012).
Gilroy, D. W., Lawrence, T., Perretti, M. & Rossi, A. G. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 3, 401–416 (2004).
Robertson, A. L. et al. A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci. Transl. Med. 6, 225ra29 (2014).
Back, M. et al. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR review 7. Br. J. Pharmacol. 171, 3551–3574 (2014).
Machado, F. S. & Aliberti, J. Role of lipoxin in the modulation of immune response during infection. Int. Immunopharmacol. 8, 1316–1319 (2008).
Romano, M. Lipoxin and aspirin-triggered lipoxins. ScientificWorldJournal 10, 1048–1064 (2010).
Romano, M., Recchia, I. & Recchiuti, A. Lipoxin receptors. ScientificWorldJournal 7, 1393–1412 (2007).
Gyurko, R. & Van Dyke, T. E. The role of polyunsaturated ω-3 fatty acid eicosapentaenoic acid-derived resolvin E1 (RvE1) in bone preservation. Crit. Rev. Immunol. 34, 347–357 (2014).
Hisada, T., Ishizuka, T., Aoki, H. & Mori, M. Resolvin E1 as a novel agent for the treatment of asthma. Expert Opin. Ther. Targets 13, 513–522 (2009).
Seki, H., Tani, Y. & Arita, M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat. 89, 126–130 (2009).
Recchiuti, A. Resolvin D1 and its GPCRs in resolution circuits of inflammation. Prostaglandins Other Lipid Mediat. 107, 64–76 (2013).
Chatterjee, A. et al. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS ONE 9, e113480 (2014).
Gong, J. et al. Maresin 1 prevents lipopolysaccharide-induced neutrophil survival and accelerates resolution of acute lung injury. Shock 44, 371–380 (2015).
Sasaki, K., Urabe, D., Arai, H., Arita, M. & Inoue, M. Total synthesis and bioactivities of two proposed structures of maresin. Chem. Asian J. 6, 534–543 (2011).
Wang, C. W. et al. Maresin 1 biosynthesis and pro-resolving anti-infective functions with human localized aggressive periodontitis leukocytes. Infect. Immun. http://dx.doi.org/10.1128/IAI.01131-15 (2015).
Lagarde, M. et al. Docosahexaenoic acid, protectin synthesis: relevance against atherothrombogenesis. Proc. Nutr. Soc. 73, 186–189 (2014).
Weylandt, K. H., Chiu, C. Y., Gomolka, B., Waechter, S. F. & Wiedenmann, B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 97, 73–82 (2012).
Pettipher, R., Hansel, T. T. & Armer, R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat. Rev. Drug Discov. 6, 313–325 (2007).
Uehara, Y. Prostaglandin D2. Nihon Rinsho 57 (Suppl.), 728–731 (in Japanese) (1999).
Uchida, K. & Shibata, T. 15-deoxy-Δ12,14-prostaglandin J2: an electrophilic trigger of cellular responses. Chem. Res. Toxicol. 21, 138–144 (2008).
Clay, C. E. et al. 15-deoxy-Δ12,14PGJ2 induces diverse biological responses via PPARγ activation in cancer cells. Prostaglandins Other Lipid Mediat. 62, 23–32 (2000).
Graham, G. J. D6/ACKR2. Front. Immunol. 6, 280 (2015).
Graham, G. J. & Locati, M. Regulation of the immune and inflammatory responses by the 'atypical' chemokine receptor D6. J. Pathol. 229, 168–175 (2013).
Chen, L., Lv, F. & Pei, L. Annexin 1: a glucocorticoid-inducible protein that modulates inflammatory pain. Eur. J. Pain 18, 338–347 (2014).
Perretti, M. & D'Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9, 62–70 (2009).
Yazid, S., Norling, L. V. & Flower, R. J. Anti-inflammatory drugs, eicosanoids and the annexin A1/FPR2 anti-inflammatory system. Prostaglandins Other Lipid Mediat. 98, 94–100 (2012).
Henderson, N. C. & Sethi, T. The regulation of inflammation by galectin-3. Immunol. Rev. 230, 160–171 (2009).
Rabinovich, G. A. & Toscano, M. A. Turning 'sweet' on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352 (2009).
Elola, M. T., Chiesa, M. E., Alberti, A. F., Mordoh, J. & Fink, N. E. Galectin-1 receptors in different cell types. J. Biomed. Sci. 12, 13–29 (2005).
Gavins, F. N., Leoni, G. & Getting, S. J. Annexin 1 and melanocortin peptide therapy for protection against ischaemic-reperfusion damage in the heart. ScientificWorldJournal 6, 1008–1023 (2006).
Perretti, M. Lipocortin 1 and chemokine modulation of granulocyte and monocyte accumulation in experimental inflammation. Gen. Pharmacol. 31, 545–552 (1998).
Qin, C. et al. Cardioprotective potential of annexin-A1 mimetics in myocardial infarction. Pharmacol. Ther. 148, 47–65 (2015).
Brzoska, T., Bohm, M., Lugering, A., Loser, K. & Luger, T. A. Terminal signal: anti-inflammatory effects of α-melanocyte-stimulating hormone related peptides beyond the pharmacophore. Adv. Exp. Med. Biol. 681, 107–116 (2010).
D'Agostino, G. & Diano, S. Alpha-melanocyte stimulating hormone: production and degradation. J. Mol. Med. (Berl.) 88, 1195–1201 (2010).
Dores, R. M. Adrenocorticotropic hormone, melanocyte-stimulating hormone, and the melanocortin receptors: revisiting the work of Robert Schwyzer: a thirty-year retrospective. Ann. NY Acad. Sci. 1163, 93–100 (2009).
Singh, M. & Mukhopadhyay, K. Alpha-melanocyte stimulating hormone: an emerging anti-inflammatory antimicrobial peptide. Biomed. Res. Int. 2014, 874610 (2014).
Bondue, B., Wittamer, V. & Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 22, 331–338 (2011).
Mariani, F. & Roncucci, L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm. Res. 64, 85–95 (2015).
Zabel, B. A. et al. Chemerin regulation and role in host defense. Am. J. Clin. Exp. Immunol. 3, 1–19 (2014).
Chung, H. T., Choi, B. M., Kwon, Y. G. & Kim, Y. M. Interactive relations between nitric oxide (NO) and carbon monoxide (CO): heme oxygenase-1/CO pathway is a key modulator in NO-mediated antiapoptosis and anti-inflammation. Methods Enzymol. 441, 329–338 (2008).
Li, L., Hsu, A. & Moore, P. K. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation — a tale of three gases! Pharmacol. Ther. 123, 386–400 (2009).
Ryter, S. W. & Choi, A. M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. 167, 7–34 (2016).
Fong, C. H. et al. An antiinflammatory role for IKKβ through the inhibition of “classical” macrophage activation. J. Exp. Med. 205, 1269–1276 (2008).
Liu, J. et al. PI3K is required for the physical interaction and functional inhibition of NF-κB by β-catenin in colorectal cancer cells. Biochem. Biophys. Res. Commun. 434, 760–766 (2013).
Rossi, A. G. L13. Apoptosis, apoptotic cell clearance and resolution of inflammation. Presse Med. 42, 536–537 (2013).
Geering, B., Gurzeler, U., Federzoni, E., Kaufmann, T. & Simon, H. U. A novel TNFR1-triggered apoptosis pathway mediated by class IA PI3Ks in neutrophils. Blood 117, 5953–5962 (2011).
El Kebir, D., Gjorstrup, P. & Filep, J. G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl Acad. Sci. USA 109, 14983–14988 (2012).
Martin, M. C., Dransfield, I., Haslett, C. & Rossi, A. G. Cyclic AMP regulation of neutrophil apoptosis occurs via a novel protein kinase A-independent signaling pathway. J. Biol. Chem. 276, 45041–45050 (2001).
Godson, C. et al. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164, 1663–1667 (2000).
Bystrom, J. et al. Resolution-phase macrophages possess a unique inflammatory and bactericidal phenotype that is controlled by cAMP. Blood 112, 4117–4127 (2008).
Sun, L. et al. Tristetraprolin (TTP)-14-3-3 complex formation protects TTP from dephosphorylation by protein phosphatase 2a and stabilizes tumor necrosis factor-α mRNA. J. Biol. Chem. 282, 3766–3777 (2007).
Fredman, G., Van Dyke, T. E. & Serhan, C. N. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler. Thromb. Vasc. Biol. 30, 2005–2013 (2010).
Rajakariar, R. et al. Nonresolving inflammation in gp91phox−/− mice, a model of human chronic granulomatous disease, has lower adenosine and cyclic adenosine 5′-monophosphate. J. Immunol. 182, 3262–3269 (2009).
Acknowledgements
The authors wish to acknowledge the Wellcome Trust and the Medical Research Council for the financial support that has enabled the research that has contributed to this field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Eicosanoids
-
Twenty-carbon structures that are derived from arachidonic acid. Examples include the prostaglandins and the leukotrienes.
- Granulocytes
-
White blood cells that are characterized by granules in their cytoplasm. These include polymorphonuclear cells, eosinophils, basophils and mast cells.
- Efferocytosis
-
The removal of effete, mainly apoptotic, cells by professional phagocytes, especially macrophages.
- Zymosan
-
A ligand found on the surface of fungi. It binds to Toll-like receptor 2 (TLR2) and dectin receptors.
- Adenine- or uridine-rich elements
-
(AREs). 3′-untranslated regions of mRNA that recruit destabilizing factors and translational silencers.
- MicroRNAs
-
(miRNAs). Small non-coding RNA molecules, containing about 21–22 nucleotides, that function by base-pairing with complementary sequences within mRNA molecules.
- Exudates
-
Mixtures of cells and fluid that accumulate outside blood vessels during inflammation.
- Non-phlogistic
-
In a non-inflammatory manner.
- Co-inhibitory molecules
-
Molecules belonging to the immunoglobulin superfamily or the tumour necrosis factor receptor superfamily that inhibit T cell receptor- mediated responses.
- Polysensitization
-
A phenomenon in which sensitization to one allergen favours sensitization to other environmental allergens.
- Macrophage plasticity
-
The ability of macrophages to alter or adapt their biological function commensurate with the inflammatory environment they inhabit.
- Stress granules
-
Sites where untranslated mRNAs accumulate in cells that have been subjected to adverse environmental conditions.
- Acantholysis
-
Pathological separation of the epidermis from the dermis.
Rights and permissions
About this article
Cite this article
Fullerton, J., Gilroy, D. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov 15, 551–567 (2016). https://doi.org/10.1038/nrd.2016.39
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2016.39
This article is cited by
-
Prognostic implications of systemic immune-inflammation index in myocardial infarction patients with and without diabetes: insights from the NOAFCAMI-SH registry
Cardiovascular Diabetology (2024)
-
15-Lipoxygenase promotes resolution of inflammation in lymphedema by controlling Treg cell function through IFN-β
Nature Communications (2024)
-
Leaf extracts of eight selected southern African medicinal plants modulate pro-inflammatory cytokine secretion in LPS-stimulated RAW 264.7 macrophages
Inflammopharmacology (2024)
-
Interface between Resolvins and Efferocytosis in Health and Disease
Cell Biochemistry and Biophysics (2024)
-
Terminalia ferdinandiana Exell. extracts reduce pro-inflammatory cytokine and PGE2 secretion, decrease COX-2 expression and down-regulate cytosolic NF-κB levels
Inflammopharmacology (2024)